CEN Study Guide often include detailed explanations and rationales, helping students grasp the underlying concepts.
Cardiovascular Emergencies CEN Study Guide
Acute Coronary Syndrome
Pathophysiology
Acute coronary syndrome (ACS) is an umbrella term for cardiac conditions in which thrombosis impairs blood flow in coronary arteries. Angina pectoris (commonly called just angina) is chest pain caused by narrowed coronary, arteries and presents with negative troponin, an ST depression, and T wave changes.
- Stable angina usually resolves in about 5 minutes. It is resolved with medications or rest and can be triggered by exertion, large meals, and extremely hot or cold temperatures.
- Unstable angina can occur at any time and typically lasts longer (> 20 minutes). The pain is usually rated as more severe than stable angina and is not easily relieved with nitrates.
- Variant angina (also called Prinzmetal angina or vasospastic angina) is episodes of angina and temporary ST elevation caused by spasms in the coronary artery. Chest pain is easily relieved with nitrates.
A myocardial infarction (Ml), or ischemia of the heart muscle, occurs when the coronary arteries are partly or completely occluded. An MI is diagnosed via positive troponin and ECG changes; it is classified by the behavior of the ST wave. A non-ST-elevation myocardial infarction (NSTEMI) includes an ST depression and a T wave inversion. A STelevation myocardial infarction (STEMI) includes an elevated ST (> 1 tntn), indicating a complete occlusion of a coronary artery.
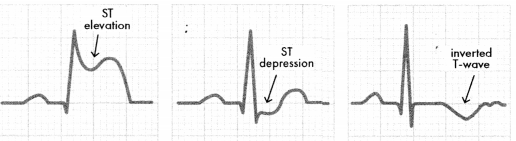
Figure 1.11. ECG Changes Associated with ACS
Signs, symptoms, and diagnostic findings for MI vary according to which coronary artery is occluded.
Septal/Anterior wall Ml is occlusion of the LAD artery, which supplies blood to the anterior of the left atrium and ventricle. Septal Ml may occur alongside anterior wall MI (but is rarely diagnosed in isolation).
- ST changes in VI - V4
- increased risk of left ventricular failure (and subsequent cardiogenic shock)
- increased risk of second-degree, type II heart block and BBB
- increased risk of ventricular septal rupture (usually 2-7 days post MI)
Inferior wall Ml is occlusion of the RCA, which supplies blood to the right atrium and ventricle, the SA node, and the AV node.
- ST changes in II, III, aVF
- presents with bradycardia and hypotension
- increased risk of AV heart blocks (because of the proximity to the SA/AV nodes)
- increased risk for papillary muscle rupture
- beta blockers and nitrates used cautiously to avoid reducing preload
Right ventricular infarction may occur with inferior wall MI.
- ST changes in V4R - V6R
- presents with tachycardia, hypotension, and JVD
- treat with positive inotropes
- avoid preload-reducing medications (beta blockers, diuretics, morphine, nitrates)
Lateral wall Ml is occlusion of the left circumflex artery, which supplies blood to the left atrium and the posterior/lateral walls of the left ventricle. Changes in ST may be seen in I, aVL, V5, or V6.
Posterior wall Ml is occlusion of the RCA or left circumflex artery, with ST elevation in V7 - V9 and ST depression in VI - V4. Posterior wall MI is rare but may be missed or read as an NSTEMI on a 12-lead ECG.
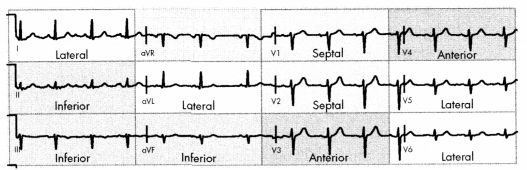
Septal/Anterior: ST changes in VI - V4
Inferior: ST changes in II, III, aVF
Right Ventricular: ST changes in V4R - V6R
Lateral-wall: ST changes in I, aVL, V5, V6
Posterior-wall: ST elevation in V7- V9 and ST depression in VI - V4
Figure 1.12. ECG Leads Changes in STEMI by Location
Physical Examination
continuous chest pain that may radiate to the back, arm, or jaw (possible Levine’s sign)
- upper abdominal pain (more common in adults > 65, people with diabetes, and women)
- dyspnea
- nausea or vomiting
- dizziness or syncope
- diaphoresis and pallor
- palpitations
Diagnostic Tests
- elevated troponin (> 0.01 ng/mL)
- elevated CK-MB (> 2.5%)
Management
An early invasive strategy with coronary angiogram is the gold standard treatment for STEMI.
- Anticipate patient transfer to the cath lab for a diagnostic evaluation with possible PCI.
- Treat with fibrinolytic agents (alteplase) ifcath lab services are not available.
- goal for door- to-balloon time: 90 minutes
- goal for door-to-fibrinolytics: 30 minutes
- Post-procedure antithrombotic therapy may include aspirin, clopidogrel, abciximab, eptifibatide, and/or heparin.
- NSTEMI is initially treated with medication (may require PCI).
- pharmacological management for ACS
- nitroglycerin
- antiplatelets: aspirin 325 mg (chewable) and platelet P2Y12
- inhibitors (e.g., clopidogrel or ticagrelor)
- supplemental oxygen
- anticoagulant (heparin or low molecular weight heparin)
- beta blocker (usually metoprolol as first choice)
- morphine (only for severe pain not relieved by nitroglycerin)
- isolated right ventricular infarction
- IV fluids
- antiplatelets and anticoagulants
- cautious use of nitrates, beta-blockers, and morphine
Aneurysm and Dissection
Pathophysiology
An abdominal aortic aneurysm (AAA), often called a triple A, occurs when the lower aorta is enlarged. Other common aneurysms include thoracic aneurysms and thoracoabdominal aortic aneurysms.
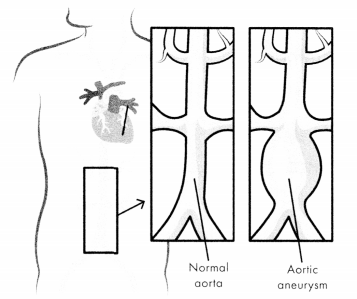
Figure 1.13. Abdominal Aortic Aneurysm (AAA)
An aortic rupture, a complete tear in the wall of the aorta, rapidly leads to hemorrhagic shock and death. An aortic dissection is a tear in the aortic intima; the tear allows blood to enter the aortic media. Both aortic rupture and dissection will lead to hemorrhagic shock and death without immediate intervention.
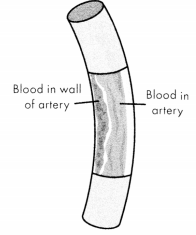
Figure 1.14. Aortic Dissection
Risk Factors
- hypertension
- heart disease
- lifestyle factors (smoking, poor diet, lack of exercise)
- genetic syndromes in which connective tissues are weakened (e.g., Marfan syndrome)
- trauma
Physical Examination
- sharp, severe pain in the chest, back, abdomen, or flank; often described as "tearing Q
- rapid, weak, or absent pulse
- a blood pressure difference of 3 > 20 mm Hg between the left and right arms
- new-onset murmur
- diaphoresis
- nausea and vomiting
- pallor
- hypotension
- orthopnea
Diagnostic Tests
- CT scan, TEE, angiogram, or chest MRI
Management
- pain management (usually morphine)
- beta-blockers; nitroprusside may also be given
- hemodynamically unstable patients: immediate surgical repair usually required
Cardiopulmonary Arrest
Pathophysiology
Ventricular tachycardia (V-tach) is tachycardia originating below the bundle of His, resulting in slowed ventricular activation. During V-tach, _> 3 consecutive ventricular beats occur at a rate > 100 bpm. V-tach is often referred to as a wide-complex tachycardia because of the width of the QRS complex.
Because the ventricles cannot refill before contracting, patients in this rhythm may have reduced cardiac output, resulting in hypotension. V-tach may be short and asymptomatic, or it may precede V-fib and cardiac arrest. Pulse-less V-tach is unstable and should be treated the same as V-fib.
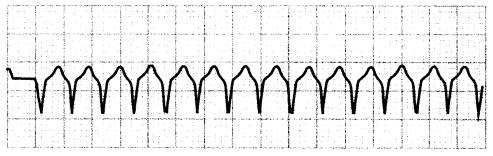
Figure 1.15. ECG: Monomorphic Ventricular Tachycardia
During ventricular fibrillation (V-fib) the ventricles contract rapidly (300- 400 bpm) with no organized rhythm. There is no cardiac output. The ECG will initially show coarse V-fib with an amplitude > 3 mm. As V-fib continues, the amplitude of the wave form decreases, progressing through fine V-fib (< 3 mm) and eventually reaching asystole.
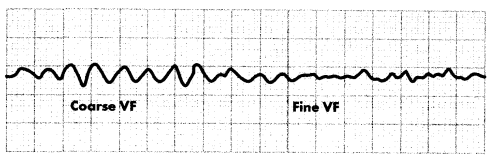
Figure 1.16. ECG: Ventricular Fibrillation
Pulseless electrical activity (PEA) is an organized rhythm in which the heart does not contract with enough force to create a pulse. Asystole, also called a "flat line,” occurs when there is no electrical or mechanical activity within the heart. Both PEA and asystole are non-shockable rhythms with a poor survival rate.
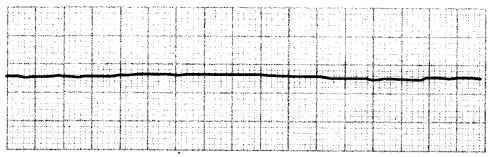
Figure 1.17. ECG: Asystole
Management
priority intervention for V-tach: check for pulse
- V-tach with a pulse, patient stable: administer amiodarone
- V-tach with a pulse, patient unstable: synchronized cardioversion
follow the advanced cardiovascular life support (ACLS) protocols for patients in pulseless V-tach, V-fib, PEA, and asystole.
Dysrhythmias
A cardiac dysrhythmia is an abnormal heartbeat or rhythm. Dysrhythmias are typically caused by a malfunction in the hearts cardiac conduction system. Most dysrhythmias of clinical importance are caused by reentry: the recitation of the heart by an electrical impulse that did not die out. Reentry dysrhythmias include A-fib, atrial flutter, V-tach, and V-fib.
Treatment is based on whether the patient is deemed hemodynamically stable or unstable.
- Stable patients can receive noninvasive interventions or drugs as a priority intervention to correct an abnormal rhythm.
- Unstable patients should receive the appropriate electrical therapy.
Bradycardia
Pathophysiology
Bradycardia is a heart rate of< 60 bpm. It results from a decrease in the sinus node impulse formation (automaticity). Bradycardia is normal in certain individuals and does not require an intervention if the patient is stable or asymptomatic. Symptomatic patients, however, need immediate treatment to address the cause of bradycardia. Symptoms of bradycardia may include hypotension, syncope, confusion, or dyspnea.
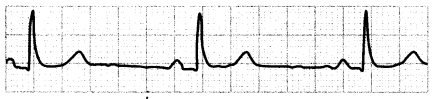
Figure 1.19. ECG: Bradycardia
Management
- stable, asymptomatic patients: monitoring with no intervention required.
- symptomatic, hemodynamically stable patients: monitor while determining underlying cause
- symptomatic, hemodynamically unstable patients: medication
- first line: atropine 0.5 mg for first dose, with a maximum of 3 mg total
- second line: dopamine or epinephrine if atropine is ineffective or if maximum dose of atropine already given and patient is still stable
- patients with bradycardia and who have had a heart transplant: administer isoproterenol (atropine is ineffective in these patients)
- unstable patients who do not respond to medication: TCP (first line), transvenous cardiac pacing (second line)
Narrow-Complex Tachycardias
Pathophysiology
Narrow-complex tachycardias (also called supraventricular tachycardias [SVT]) are dysrhythmias with > 100 bpm and a narrow QRS complex (< 0.12 seconds). The dysrhythmia originates at or above the bundle of His (supra¬ ventricular), resulting in rapid ventricular activation. Specific SVT rhythms include AV nodal reentrant tachycardia(AVNRT), AV reentrant tachycardia (AVRT), and atrial tachycardia (AT).
Narrow-complex tachycardias are often asymptomatic. Symptomatic patients may have palpitations, chest pain, hypotension, and dyspnea.
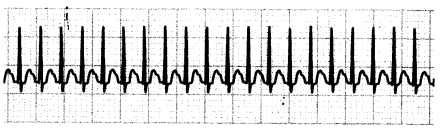
Figure 1.20. ECG: Supraventricular Tachycardia
Management
- first line: vagal maneuvers
- second line: medication
- rapid bolus dose of adenosine 6 mg
- second dose of adenosine 12 mg given if chemical cardioversion does not occur within1-2 minutes
- refractory SVT
- stable patients: calcium channel blockers (e.g., diltiazem), beta-blockers, or digoxin
- unstable patients and patients for whom medications are ineffective: synchronized cardioversion
Atrial Fibrillation and Flutter
Pathophysiology
Atrial fibrillation (A-fib) is an irregular narrow-complex dysrhythmia. During A-fib, the atrium does not contract normally, which may cause blood clots to form in the left atrium (on the left atrial appendage), increasing stroke risk. The irregular atrial contractions also decrease cardiac output.
In atrial fibrillation with rapid ventricular response (A-fib with RVR), the ventricular rate is > 100 bpm. The ECG in A-fib will show an irregular ventricular rhythm with no clear P waves and an undeterminable atrial rate.
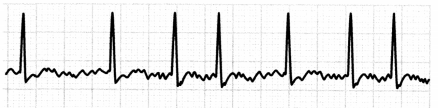
Figure 1.21. ECG: Atrial Fibrillation
Duringatrial flutter, the atria beat regularly but too fast (240- 400 bpm), causing multiple atrial beats in between the ventricular beat. Atrial flutter can be regular or irregular. The ECG in atrial flutter will show a saw-toothed flutter and multiple P waves for each QRS complex.
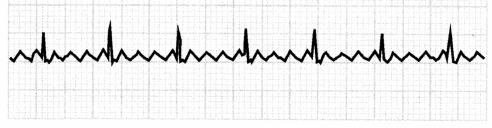
Figure 1.22. ECG: Atrial Flutter
Management
- adenosine: slows the rhythm so that it may be identified (i.e., discern between atrial dysrhythmia and SVT), but will not convert dysrhythmia to a sinus rhythm
- hemodynamically stable patients: medication
- calcium channel blockers (diltiazem), beta-blockers, or cardiac glycoside to slow the rhythm
- antidysrhythmics (amiodarone) to convert to sinus rhythm
- hemodynamically unstable patients: cardioversion
- anticoagulation to lower risk of stroke
- cardiac ablation may be used to correct A-fib and atrial flutter
Myocardial Conduction System Defects
ATRIOVENTRICULAR BLOCKS
Pathophysiology
An atrioventricular (AV) block is the disruption of electrical signals between the atria and ventricles. The electrical impulse may be delayed (first-degree block), intermittent (second-degree block), or completely blocked (third-degree block).
A first-degree AV block occurs when the conduction between the SA and the AV nodes is slowed, creating a prolonged PR interval. A first-degree AV block is a benign finding that is usually asymptomatic, but it can progress to a second-degree or third-degree block.
The ECG in a first-degree AV block will show a prolonged PR interval of > 0.20 seconds.
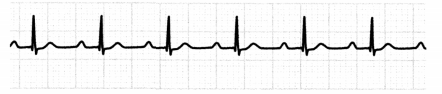
Figure 1.23. ECG: First-Degree Atrioventricular (AV) Block
A second-degree AV block, type1 (Wenckebach or Mobitz type 1), occurs when the PR interval progressively lengthens until the atrial impulse is completely blocked and does not produce a QRS impulse. This dysrhythmia occurs when the atrial conduction in the AV node or bundle of His is either slowed or blocked. This type of block is cyclic; after the dropped QRS complex, the pattern will repeat itself.
The ECG in second-degree AV block, type 1, will show progressively longer PR intervals until a QRS complex completely drops.
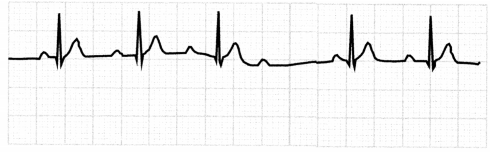
Figure 1.24. ECG: Second-Degree AV Block, Type 1
A second-degree AV block, type 2 (Mobitz type 2), occurs when the PR interval is constant in length but not every P wave is followed by a QRS complex. This abnormal rhythm is the result of significant conduction dysfunction within the His-Purkinje system.
The ECG in second-degree AV block, type 2, will show constant PR intervals and extra P waves, with dropped QRS complexes.
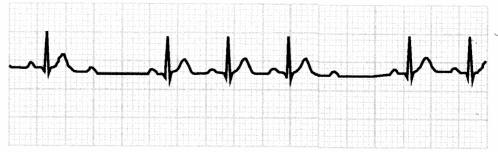
Figure 1.25. ECG: Second-Degree AV Block, Type 2
A third-degree AV block, sometimes referred to as a complete heart block, is characterized by a complete dissociation between the atria and the ventricles. There are effectively 2 pacemakers within the heart, so there is no correlation between the P waves and the QRS complexes. The most common origin of the block is below the bundle of His, but the block can also occur at the level of the bundle branches of the AV node.
The ECG for the third-degree AV block will show regular P waves and QRS complexes that occur at different rates. There will be more P waves than QRS complexes, with P waves possibly buried within the QRS complex.
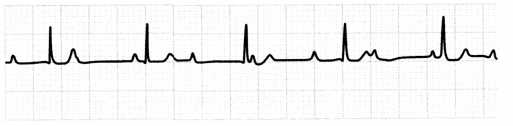
Figure 1.26. ECG: Third-degree AV Block
Physical Examination
- first- and second-degree AV blocks usually asymptomatic
- may show symptoms of reduced cardiac output (e.g., hypotension, dyspnea, chest pain)
- bradycardia
Management
- symptomatic patients: TCP possibly needed to manage symptoms
- implantable pacemaker if underlying cause cannot be resolved
- hypotensive patients: dopamine or epinephrine may be needed
- discontinue medications that slow electrical conduction in the heart (e.g., antidysrhythmic, beta-blockers)
Bundle Branch Block
Right bundle branch block (RBBB) and left bundle branch block (LBBB) are interruptions in conduction through a bundle branch.
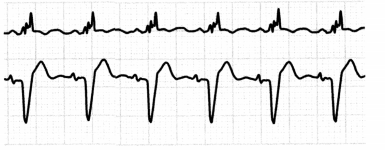
Figure 1.27. ECG: Bundle Branch Blocks
- Ischemic heart disease is the most common cause of both RBBB and LBBB.
- LBBB can also arise from other heart diseases, hypertension, digoxin, or cardiomyopathy.
- Other causes of RBBB include cor pulmonale, pulmonary embolism, and COPD.
- Both RBBB and LBBB may occur in the absence of heart disease.
- LBBB in particular is associated with progressive underlying structural heart disease and is associated with poor outcomes post-MI.
- If the patient with a BBB is asymptomatic, no treatment is necessary.
- Patients with syncopal episodes may need to have a pacemaker inserted.
Congenital Conduction Defects
- Long QT syndrome is a cardiac electrical disturbance that causes a prolonged ventricular repolarization (seen as a QT interval > 0.44 seconds on ECG).
- may be asymptomatic or present with dysrhythmias (especially torsades de pointes), syncope, seizure, or sudden cardiac death
- management: beta-blockers may be used; placement of an ICD; stop medications likely to prolong the QT interval
- Wolff-Parkinson-White syndrome, caused by an early excitation of an extranodal accessory pathway, results in tachycardia.
- Patient may be asymptomatic or present with sudden A-fib or paroxysmal tachycardia with HR > 150.
- ECG shows short PR interval (< 0.12 seconds) with a slurred QRS upstroke and a wide QRS (> 0.12 seconds).
- Treatment is synchronized cardioversion. Unstable patients may require catheter ablation.
- Contraindicated medications include adenosine, digoxin, amiodarone, beta-blockers, and calcium channel blockers.
- Brugada syndrome is a genetically inherited cardiac electrical pathway syndrome that is linked to 4% - 12% of all sudden cardiac deaths.
- It is diagnosed by characteristic ECG findings with sudden cardiac arrest, ventricular tachydysrhythmias, or syncopal episodes.
- The ECG shows pseudo-RBBB and persistent ST-segment elevation.
- The ECG abnormalities may be unmasked by sodium channel blockers.
- It is treated with medication (quinidine or flecainide) or ICD placement.
- Medications likely to prolong the QT interval are contraindicated.
Endocarditis
Pathophysiology
Infective endocarditis occurs when an infection causes inflammation of the endocardium. The inflammation impairs valve function and may also disrupt the electrical conduction system. Infective endocarditis can occur secondary to surgical valve replacement; these cases have a high mortality rate.
Risk Factors
- more common in women and immunocompromised individuals
- heart valve problems or surgery involving heart valves
- poor dental health or recent dental work
- central venous line access
- rheumatic heart disease
- history ofillegalIV drug use
Physical Examination
- chest pain
- flu-like symptoms (chills, fatigue)
- fever
- murmur
- petechiae
- Janeway lesions
- Osier's nodes
- Roth spots
- joint pain
- dyspnea
- splinter hemorrhages under fingernails
- hematuria
- night sweats
- weight loss
Diagnostic Tests
- blood cultures showing bacteria present in the bloodstream
- WBC; may be elevated because of infection
- elevated C-reactive protein and sedimentation rate due to inflammation
- echocardiogram and TEE showing any possible heart valve damage and vegetation
Management
- aggressive treatment with IV antimicrobials
- pharmacologic management of symptoms: antipyretics, diuretics, or dysrhythmias
- surgical repair of valves if necessary
Heart Failure
Pathophysiology
Heart failure (HF) is a clinical syndrome caused by a variety of pathophysiologic processes; it is important to know the cause (i.e., ischemic, infiltrative, congenital) for better long-term management. Acute decompensated heart failure is the sudden onset or worsening of HF symptoms usually manifested by fluid volume overload.
HF is classified according to the left ventricular ejection fraction. Impairment of systolic function results in heart failure with reduced ejection fraction (HFrEF, or systolic HF), classified as an ejection fraction of< 50%. Heart failure with preserved ejection fraction (HFpEF, or diastolic HF) is characterized by an ejection fraction of > 50% and diastolic dysfunction.
HF can also be categorized as left-sided or right-sided, depending on which ventricle is affected. Left-sided HF is usually caused by cardiac disorders (e.g., MI, cardiomyopathy) and produces symptoms related to pulmonary function. Right-sided HF is caused by right ventricle infarction or pulmonary conditions (e.g., PE, COPD) and produces symptoms related to systemic circulation. Unmanaged left-sided HF can lead to right-sided HF.
Risk Factors
- coronary artery disease, history of MI, and/or hypertension
- lifestyle factors (smoking, poor diet, lack of exercise)
- diabetes
- obesity
- congenital heart disease
Physical Examination
Left-Sided HF: increased pulmonary capillary, wedge pressure, increased PAP, dyspnea or orthopnea, pulmonary edema, tachycardia, bibasilar crackles, cough, frothy sputum, hemoptysis, left-sided S3 sound, diaphoresis, pulsus alternans.
Right-Sided HF: increased right ventricular end-diastolic, pressure (RVEDP) and right atrial pressures, increased CVP, increased PAP, dependent edema (usually in lower legs); ascites, JVD, hepatomegaly, right-sided S3 sound, weight gain, nausea, vomiting, abdominal pain, nocturia.
Diagnostic Tests
- BNP > 100 pg/mL
- echocardiogram to assess ejection fraction, ventricular hypertrophy, valve dysfunction
- CXR to show cardiomegaly or pulmonary congestion
- right heart catheterization to assess filling pressures and hemodynamics
Management
- Pharmacological, dietary, and surgical interventions vary depending on the patient s type and degree of HF.
- Managing decompensated HF:
- Treatment goal is to improve cardiac output, Cl, and respiratory status.
- Administer supplemental oxygen (e.g., nasal cannula, BIPAP).
- Keep patient upright (sitting or semi-Fowler’s position).
- First-line medication is a loop diuretic (e.g., furosemide or bumetanide).
- If severe, reduce afterload and preload with nitroprusside or hydralazine.
- If Cl remains low, start inotropes (e.g., milrinone, dopamine).
- Consider IABP placement if severely decompensated.
- For hemodynamically stable patients, start or resume medication (e.g., ACE-Is, ARBs, beta blockers).
- Patient should be on fluid/salt restriction.
- ICD/pacemaker, VAD, or heart transplant are long-term possible interventions.
Hypertensive Crisis
Pathophysiology
Hypertensive crises include hypertensive urgency and hypertensive emergencies. Hypertensive urgency occurs when blood pressure is > 180/110 mm Hg without evidence of organ dysfunction. A hypertensive emergency occurs when systolic blood pressure is > 180 mmHg or when diastolic blood pressure is >120 mm Hg and when either of these is accompanied by evidence of impending or progressive organ dysfunction (e.g., elevated kidney function, abnormal liver enzymes). Hypertensive crises increase the risk of cerebral infarction, and prolonged hypertension can lead to heart or renal failure.
Risk Factors
- history of hypertension (particularly with medication noncompliance or inadequate treatment)
- kidney disease
- endocrine conditions (e.g., Cushing disease)
- use of sympathomimetic drugs (e.g., amphetamines)
Physical Examination
- headache
- blurred vision
- dizziness
- dyspnea
- retinal hemorrhages
- epistaxis
- chest pain
Management
- blood pressure reduction
- limited to a decrease of< 25% within the first 2 hours to maintain cerebral perfusion
- goal of SBP of140 - 160 mm Hg or DBP less than 105 mm Hg
- first-line medications: IV antihypertensives (e.g., labetalol, hydralazine, nicardipine)
- quiet, non-stimulating environment
- O2 administration
Cardiac Tamponade
Pericarditis is the inflammation of the pericardium, the lining that surrounds the heart. When inflammation occurs, fluid can accumulate, resulting in pericardial effusion.
Pericardial tamponade (also called cardiac tamponade) occurs when the effusion is large enough to impair the ability of the heart to pump blood sufficiently. The increased pressure reduces chamber compliance and filling. With enough pressure, venous return is reduced and cardiac output drops, causing hemodynamic compromise.
The onset of cardiac tamponade may be acute (usually due to trauma) or subacute. The most common etiologies of subacute cardiac tamponade are infection, MI, and malignancy.
Symptoms and Physical Findings
- Beck’s triad
- low arterial BP
- dilated neck veins
- muffled heart sounds
- sudden and severe chest pain
- increases with movement, lying flat, and inspiration
- decreases by sitting up or leaning forward
- tachycardia (usually the earliest sign)
- hypotension
- pulsus paradoxus
- pericardial rub
- dyspnea
Diagnostic Tests
- ECG
- ST elevation possible, usually in all leads except aVR and VI
- tall, peaked T waves
- CXR showing “water bottle” silhouette in pericardial effusion.
- An echocardiogram may show pericardial effusion, thickening, or calcifications.
Management
- definitive treatment: pericardiocentesis or surgical drainage
- Hemodynamically stable patients may be monitored while underlying condition is treated:
- maintain fluid volume
- manage pain (positioning, analgesics)
Peripheral Vascular Disease
- Atherosclerosis, also called atherosclerotic cardiovascular disease (ASCVD), is a progressive condition in which plaque builds up in the tunica intima of arteries.
- The presence of advanced atherosclerosis places patients at a high risk for several cardiovascular conditions, including stenosis, aneurysms, and arterial/venous occlusions.
- Atherosclerosis can occur in any artery and is categorized according to the location of the plaque buildup.
- peripheral vascular disease (also called peripheral artery disease): narrowing of the peripheral arteries
- coronary artery disease (CAD): narrowing of the coronary arteries
- renal artery stenosis: narrowing of the renal arteries
- carotid artery occlusive disease (CAOD), or stenosis: narrowing or hardening of the carotid arteries.
Risk factors for atherosclerosis include:
- age (some degree of atherosclerosis is common in people > 65)
- dyslipidemia
- hypertension
- lifestyle factors (smoking, poor diet, lack of exercise)
- obesity
- diabetes
Thromboembolic Disease
ARTERIAL OCCLUSION
Pathophysiology
Acute peripheral vascular insufficiency (also acute arterial occlusion) occurs when a thrombus or an embolus occludes a peripheral artery, causing ischemia. This condition is a medical emergency requiring prompt treatment to prevent tissue necrosis.
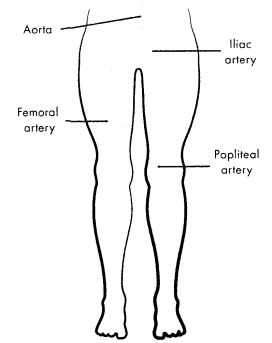
Figure 1.28. Common Locations of Acute Arterial Occlusion
Physical Examination
- the 6 Ps (hallmark signs) of an arterial occlusion
- pain (intermittent claudication)
- pallor
- pulselessness
- paresthesia
- paralysis
- poikilothermia
- petechiae (visible with microemboli)
- ankle-brachial index (ABI) < 0.30: indicates low likelihood of limb survivability
Diagnostic Tests
- duplex ultrasonography, CT angiography, or catheter-based arteriography
- elevated D-dimer
Management
- medications: IV anticoagulants (usually heparin), thrombolytics, and/ or antiplatelet agents
- surgical/catheter intervention: catheter-directed thrombolysis, bypass surgery, or embolectomy
- nursing considerations
- frequent pulse and neurovascular checks
- do not elevate extremity
- monitor for signs and symptoms of bleeding following use of anticoagulants or thrombolytic
Venous Occlusion
Pathophysiology
A deep vein thrombosis (DVT) is the most common form of acute occlusion and occurs when a thrombus forms within a deep vein. DVT is most common in the lower extremities.
Risk Factors
- Virchow’s triad
- hypercoagulability (e.g., due to estrogen, contraceptive use, or malignancy)
- venous stasis (bed rest or any other activity that results in decreased physical movement)
- endothelial damage (damage to the vessel wall from trauma, drug use, inflammatory processes, or other causes)
- pregnancy, hormone replacement therapy, or oral contraceptives
- recent surgery
Physical Examination
- pain localized to a specific area (usually the foot, ankle, calf, or behind the knee)
- unilateral edema, erythema, and warmth
- positive Homan's sign
Diagnostic Tests
- elevated D-dimer
- venous duplex ultrasonography
Management
- pharmacological management: anticoagulants (first line), thrombolytics (second line)
- surgical or endovascular thrombectomy (if medication is ineffective or contraindicated)
- inferior vena cava (IVC) filter: may be placed to avoid a PE in patients who cannot tolerate anticoagulants
Cardiac Trauma
- Cardiac trauma occurs when an outside force causes injury to the heart. Cardiac trauma can cause rupture of heart chambers, dysrhythmias, damage to the heart valves, or cardiac arrest.
- Blunt cardiac injury (BCI) occurs when an object forcefully strikes the chest. Damage due to BCI may be caused by compression of the heart between the sternum and spine, pressure fluctuations in the thoracic cavity, or shearing forces. Because the right side of the heart is anteriorly positioned, it is typically the most affected.
- Cardiac contusion is a general term used to describe damage to the heart from blunt trauma.
- Common consequences of BCI include dysrhythmias; damage to chamber walls, septa, or valves; and decreased contractility and SV.
- Management of BCI may include antidysrhythmic drugs, temporary pacemakers, medications to manage heart failure, and surgery to repair damaged heart tissues.
- Fluid and electrolytes should be monitored closely to preserve my cardial conduction and cardiac output.
- Blunt aortic injury (BAI) is a tear in the aorta resulting from compression of the aorta between the vertebrae and anterior chest wall. Patients with BAI are administered antihypertensives (sodium nitroprussideIV infusion) to maintain SBP < 90 mm HG and usually require surgery.
- Penetrating cardiac trauma involves the puncture of the heart by a sharp object or a broken rib. The penetration causes blood to leak into the pericardial space or mediastinum.
- The most frequently affected area is the right ventricle (due to its anterior position).
- Blood leakage can result in cardiac tamponade, and blood loss from penetrating injuries can also result in shock.
- Penetrating objects should be stabilized and the patient prepped for surgery.
Cardiogenic Shock
Pathophysiology
Cardiogenic shock is a cyclical declinein cardiac function. It results in decreased cardiac output, impaired oxygen delivery, and reduced tissue perfusion. A lack of coronary perfusion causes or escalates ischemia/infarction by decreasing the ability of the heart to pump effectively. HR increases to meet myocardial oxygen demands. However, the reduced pumping ability of the heart decreases cardiac output. Demands for coronary or tissue perfusion are not met. LVEDP increases, leading to stress in the left ventricle and an increase in afterload. This distress results in lactic acidosis.
Cardiogenic shock is most common after an MI but can be associated with trauma, pericardial tamponade, or dysrhythmia.
Physical Examination
- tachycardia and sustained hypotension (SBP < 90 mm Hg)
- oliguria (< 30 mL/hr or < 0.5 mL/kg/hr output)
- crackles
- tachypnea and dyspnea
- pallor
- JVD
- altered LOC
- cool, clammy skin
- S3 heart sound possible
Diagnostic Tests
- CI < 2,2 L/min/m2
- PAOP > 15 mm Hg
- elevated SVR, CVP, PCWP
- MAP < 60 mm Hg
- elevated lactate
- ABG shows metabolic acidosis and hypoxia
Management
- immediate goal: reduce cardiac workload and improve myocardial contractility
- initial, careful administration of intravenous fluids
- medications given based on hemodynamic status
- vasopressor (usually norepinephrine) for hypotensive patients
- inotrope (usually dobutamine) and vasodilator for normotensive patients
- other interventions
- IABP to reduce afterload and increase coronary perfusion
- cardiac catheterization with PCI (if cause is thought to be ischemic) to improve myocardial perfusion
- LVAD
- monitor patient for cardiac dysrhythmias
Obstructive Shock
Pathophysiology
Obstructive shock is reduced cardiac output caused by extracardiac mechanical obstruction or compression of the vasculature. Obstruction of the pulmonary vasculature (e.g., pulmonary embolism, pulmonary hypertension) increases pulmonary vascular resistance and decreases cardiac output. Compression of the heart (e.g., tension pneumothorax, pericardial tamponade) prevents atrial filling, reducing preload and cardiac output.
Physical Examination
- hypotension (may initially be moderate)
- visibleJVD
- nonspecific signs and symptoms of shock
- tachycardia
- oliguria
- tachypnea or dyspnea
- altered mental status
- cool, mottled extremities
Diagnostic Tests
- ECG and CXR
- CT or ultrasound based on suspected etiology
Treatment and Management
- immediate management of underlying condition
- IV fluids and vasopressors as needed
Read More